Hydrogen and oxygen can be combined in a fuel cell to produce electrical energy. A fuel cell uses a chemical reaction to provide an external voltage, as does a battery, but differs from a battery in that the fuel is continually supplied in the form of hydrogen and oxygen gas. It can produce electrical energy at a higher efficiency than just burning the hydrogen to produce heat to drive a generator because it is not subject to the thermal bottleneck from the second law of thermodynamics. It's only product is water, so it is pollution-free. All these features have led to periodic great excitement about its potential, but we are still in the process of developing that potential as a pollution-free, efficient energy source (see Kartha and Grimes).
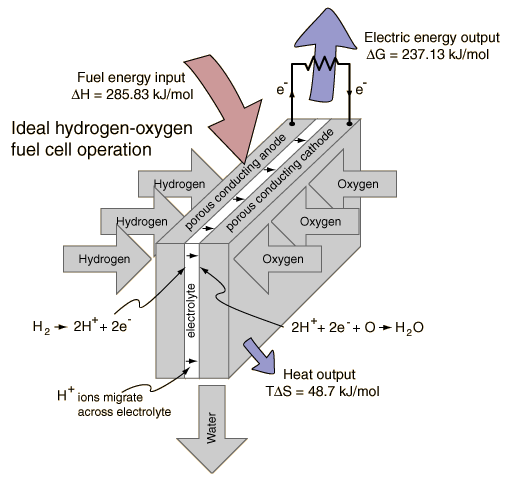
Combining a mole of hydrogen gas and a half-mole of oxygen gas from their normal diatomic forms produces a mole of water. A detailed analysis of the process makes use of the thermodynamic potentials. This process is presumed to be at 298K and one atmosphere pressure, and the relevant values are taken from a table of thermodynamic properties.
| Quantity | ||||
| Enthalpy | ||||
| Entropy |
Energy is provided by the combining of the atoms and from the decrease of the volume of the gases. Both of those are included in the change in enthalpy included in the table above. At temperature 298K and one atmosphere pressure, the system work is
Since the enthalpy H= U+PV, the change in internal energy U is then
The entropy of the gases decreases by 48.7 kJ in the process of combination since the number of water molecules is less than the number of hydrogen and oxygen molecules combining. Since the total entropy will not decrease in the reaction, the excess entropy in the amount TΔS must be expelled to the environment as heat at temperature T. The amount of energy per mole of hydrogen which can be provided as electrical energy is the change in the Gibbs free energy:
For this ideal case, the fuel energy is converted to electrical energy at an efficiency of 237.1/285.8 x100% = 83%! This is far greater than the ideal efficiency of a generating facility which burned the hydrogen and used the heat to power a generator! Although real fuel cells do not approach that ideal efficiency, they are still much more efficient than any electric power plant which burns a fuel.
In comparing the fuel cell process to its reverse reaction, electrolysis of water, it is useful treat the enthalpy change as the overall energy change. The Gibbs free energy is that which you actually have to supply if you want to drive a reaction, or the amount that you can actually get out if the reaction is working for you. So in the electrolysis/fuel cell pair where the enthalpy change is 285.8 kJ, you have to put in 237 kJ of energy to drive electrolysis and the heat from the environment will contribute TΔS=48.7 kJ to help you. Going the other way in the fuel cell, you can get out the 237 kJ as electric energy, but have to dump TΔS = 48.7 kJ to the environment.
Explore Fuel Cells
In another project, Electrolysis: Obtaining hydrogen from water: The Basis for a Solar-Hydrogen Economy, we have discussed how hydrogen might be used as a clean burning fuel, and how it can be produced cleanly from water. Generating heat, however, is not always the best thing to do, because entropy is created when heat is generated, and that can limit the efficiency of devices that use that heat energy to do useful work (See the section on entropy in our Energy Physics Primer).
Fortunately, there exists a device called a fuel cell, which can chemically combine hydrogen with oxygen to make electricity without involving heat (although some heat is usually generated in practical situations)! Here is a fuel under development by Manhattan Scientifics:

This is actually a stack of fuel cells: Each fuel cell by itself doesn't produce very much power, but the voltage provided by each fuel cell individually adds up, yielding a voltage (and a power) that is large enough for practical applications.
Fuel cells can be used for electrolysis as well - splitting water into hydrogen and oxygen, so that the hydrogen can be stored as a fuel. NMSEA has some a fuel cell demonstration unit which has two fuel cells: one cell makes hydrogen from water using solar electricity - the other fuel cell converts the hydrogen into electricity to power a small fan. Thus, the whole principle of a hydrogen economy is demonstrated in a single unit.
What are the advantages of fuel cells?
- No moving parts
- Reliable
- Efficient (50%-90% presently): This is major long-term advantage - fuel cells are not limited by the thermodynamics constraints that heat-based combustion type processes are subject to.
- Heat generated can be captured for other uses
- Operates cleanly (emits only water)
- Quiet
What are the disadvantages of fuel cells?
- Platinum catalysts are still expensive (but much less so than a decade ago due to the approach of depositing platinum particles on carbon - see below).
When will they become widespread?
Although the principle of fuel cells was discovered in 1839 (by Sir William Grove, the "Father of the Fuel Cell"), and the first practical cells developed in the 1930's, fuel cells have not yet found widespread use. They have found use in applications in closed environments such as space technology and submarines where cost is not an issue. They will likely make their first widespread appearance in particular applications such as:
- Common devices such as laptop computers and vacuum cleaners; mid-scale applications where a mobile source of electricity is required. Fuel cell cars may appear sometime between 2005 and 2015. A hydrogen fuel cell bus fleet was recently tested in Chicago, and performed very well.
- To power facilities such as hospitals that need a source of non interruptable power.
Examples
Here is a photo off a fuel cell bike under development by Manhattan Scientifics: the fuel cell is mounted on the steering column, and the hydrogen tank can be seen jutting out over the rear wheel. The rear axel is turned by an electric motor powered by the fuel cell:


Fuel Cell History
The principle of the fuel cell was discovered in 1839 by Sir William Grove, acknowledged as the "Father of the Fuel Cell". Grove was interested in reversing the process of electrolysis - precisely what a fuel cell achives. The term "fuel cell" was coined in 1889 by Ludwig Mond and Charles Langer, who attempted to use air and coal gas to generate electricity. In 1932, Francis Bacon improved on the platinum catalysts of Mond and Langer, and soon Harry Karl Ihrig, of Allis-Chalmers Manufacturing Company demonstrated a 20-horsepower fuel cell powered tractor. NASA began using fuel cells in the late 1950s and continues to do so today.
How do they work?
The process by which the hydrogen is combusted (burned) in the presence of oxygen is
2H2 + O2 -> 2 H2O + energy (heat).
The process for fuels cells is very similar, except that this time we get electricity instead of heat:
2H2 + O2 -> 2 H2O + energy (electricity)
One fuel cell type, called a proton exchange membrane (PEM) fuel cell, carries out the reaction above in the following way:

The hydrogen fuel (H2) enters one side of the fuel cell, where it encounters a catalyst, for example platinum, which splits the hydrogen atoms into a proton (H+) and electron (e-). The proton then travels through a membrane (the proton exchange membrane) to the other side of the fuel cell. But the electron cannot permeate easily through the membrane. Instead, its travels through an electrical wire to get to the other side, and delivers its energy to a "load" along the way, such as a light bulb. When it gets the other side of the fuel cell, the electron is recombined with the proton and the electron and an oxygen molecule from the air to make water.
The membrane in a PEM cell is made from "nafion", a sulfinate polymer made by Dupont. This only lets protons through because there are sulfinate (SO4) molecules in the polymer, which contain oxygen. The oxygen atoms "hog" the electrons of the sulfinate molecules, making the oxygen atoms slightly negatively charged, such that the positively charged protons can weakly bind to them. This allows them to permeate the membrane, and jump from one sulfinate molecule to another across the membrane, with help from thermal fluctuations and the electric field created across the membrane by the electron flow.

Tidak ada komentar:
Posting Komentar